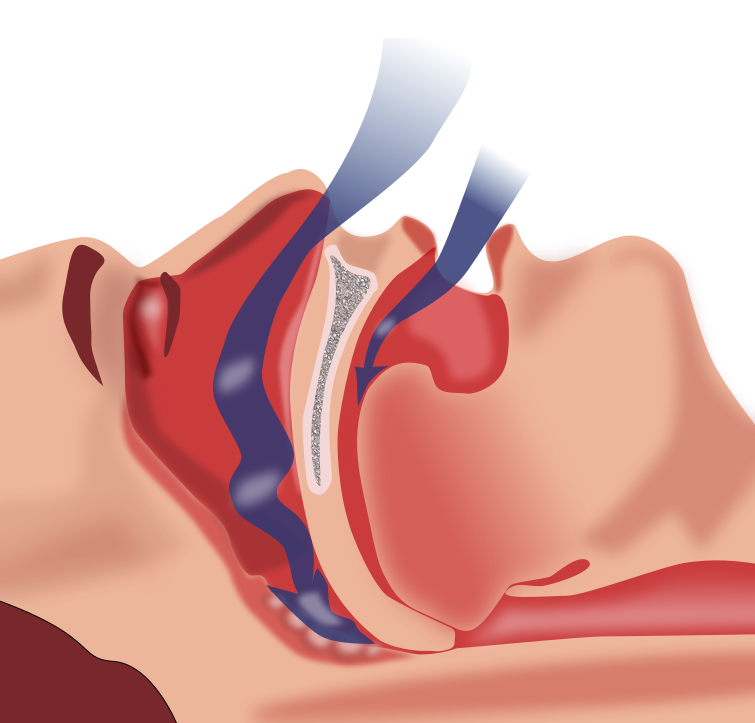
A friend of mine died a couple of years back, of heart failure. Heart problems are very common, anid have many possible causes. He also had a condition called obstructive sleep apnoea, which has long been linked with heart problems. But nobody's ever quite known why.
Obstructive sleep apnoea, or OSA, is surprisingly common, affecting almost a quarter of men and almost one in ten women. The muscles and soft tissues of the throat relax and collapse, blocking the airway wholly or partially and therefore interrupting breathing.
Now, for sleep to serve its purpose, we have to spend a certain amount of time in a state of deep sleep. Each time you have an episode of OSA, you enter lighter sleep or even wake very briefly in order to restore normal breathing. This cycle can repeat many times a night, up to once a minute in extreme cases.
These repeated sleep interruptions lead to the person with OSA feeling very tired during the day. They have no memory of the periods of breathlessness, so are often unaware that they are not sleeping properly.
Among the complications of OSA are heart problems: increased heart rate and blood pressure, and heart attack. The mechanism by which this happens has never been understood. Now a group of researchers at George Washington University in Washington DC seem to have uncovered it.
Our resting heart rate is maintained at an appropriately low level by a group of parasympathetic neurons in the brainstem. By mimicking OSA in rats, the researchers discovered that during OSA episodes the activity of these neurons is inhibited, leading to an increase in heart rate and the possibility of irregular heartbeat and high blood pressure.
It's too late for my friend David. But now researchers know where to focus their future work. They must try to restore the normal cardio-protective function of these neurons in people with OSA, to reduce the risk of cardiac problems, so that OSA no longer carries the risk of death.
Showing posts with label research. Show all posts
Showing posts with label research. Show all posts
Friday, 16 May 2014
Friday, 27 July 2012
MS research roundup July 2012
Another research roundup, and so soon! But there's just been so much interesting research to tell you about.
We already know that someone's risk of getting MS is linked to where they were brought up: very roughly, the further away from the equator, the higher the risk. Another known risk factor is having antibodies to Epstein-Barr virus (EBV): people with MS are far more likely to have EBV antibodies in their blood than people without.
British researchers investigated the distribution of EBV infection, and found it was the same as for MS: infection was more common further away from the equator. We still have a lot to learn about what causes MS, but this could be a piece in the puzzle.
I've often written before about Vitamin D. Its importance is showing up over and over again in any number of different conditions, including MS. Now an Australian study is under way, to see whether taking Vitamin D can prevent MS. The trial will test 3 dosage levels of Vitamin D against placebo in people who've had a first attack of MS-like symptoms, and will run till 2016. It'll be interesting to see the results.
All of us with MS are different: each of us has different symptoms and a different disease course. We can look at averages, though. American researchers studied over 1000 people with MS. They found that RRMS does not inevitably develop into SPMS, and the onset of progression is more dependent on age than on how long the person has had MS.
As neurons are damaged by MS, cell metabolism is disrupted and there is a buildup of sodium. British and French researchers found that sodium buildup measured by MRI could indicate the degree of MS progression. In those with more advanced disease, sodium buildup in the motor cortex is correlated with greater levels of disability.
This is early-stage research, but could become really useful in monitoring disease progression. With advanced disease, sodium was raised even in apparently normal white matter.
A group of researchers in Germany have discovered an antibody found in the blood of about half of people with MS that is not found in people without the condition. The antibody's implications aren't fully understood as yet, but in rodents it binds to and damages brain cells known to be important for neurological function.
In time this may lead to a blood test for MS and/or a treatment preventing the antibody from binding to brain cells.
Researchers in America have used monoclonal antibodies to stimulate myelin repair in mice with a condition similar to MS. Similar work had been done before, but using far larger molecules than in this research.
They found the molecules were effective, and quick and cheap to synthesise. They were also stable and unlikely to cause an immune response. Much more work needs to be done, but this could in time be a suitable candidate for human trials.
The nerve damage in MS is caused by T-cells, part of the immune system. American researchers are about to start clinical trials using an extract of sea anemone venom, which they've found is a highly selective suppressant of T-cell activity. They hope it should prevent further damage to the nerves.
It's already known that fingolimod (Gilenya / Novartis) reduces relapse rates and disease progression. Swiss researchers have now shown that it's also associated with a reduction in the number of lesions, and slowing of the rate of brain volume loss. Not everyone is able to have fingolimod, but for those who can, this is just another benefit of another hugely useful treatment.
Modafinil (Provigil) is approved to treat excessive daytime sleepiness in narcolepsy and some similar conditions. It is also used for fatigue and sleepiness in MS. In a small trial, people taking modafinil had no significant change in disability level over 3 years, while those not on modafinil did have significant changes.
A much larger study is needed, and particularly comparisons made between taking modafinil with different disease modifiers. This is a really interesting result though,with a lot of potential.
Taiwanese researchers carried out a meta-analysis of studies on the effect of cranberry juice on UTIs. The results confirmed that cranberries do protect against UTIs. The effect was particularly strong in women with recurrent UTIs. There were,however, concerns about the amount of sugar in cranberry juice.
Finally, some excellent news: Canadian researchers have found that people with MS are less likely to get cancer than the general population, particularly bowel cancer. The risk level varied for different types of cancer.
Further studies are needed to find out why people with MS have these different levels of cancer: it may be because both MS and cancer involve the immune system. Still, good news is good news!
Sunday, 24 June 2012
MS Research Roundup June 2012 Part 2
This is the second part of the current research roundup. Part 1 is here. In this part, I'll be looking at MS treatments.
The symptoms of MS are caused by damage to the fatty myelin sheath round nerve cells. American researchers found that the immune systems of people with MS were targeting 4 particular lipids (fats). In autopsy, these lipids were depleted in areas of MS damage.
Working with mice with an MS-like illness, they then injected the lipids over several weeks. Disease severity was limited and even reversed. Some tests now show that the mice can take the lipids orally and still improve.
This research is at very early stages, but it holds huge promise for a treatment that can actually repair our damaged myelin.
Omega-3 fatty acids are thought to be helpful for many conditions, but not much work has been done on how they work. American scientists fed three types of fatty acid to mouse macrophages, which they stored as phospholipids. The scientists then stimulated the cells to produce inflammatory responses.They found that Omega-3 inhibited an enzyme called cyclooxygenase (COX), which produces the prostaglandin hormones that spark inflammation.
Inflammation is part of the process of damage to nerve cells that leads to MS symptoms. This research is a big clue as to what could be happening inside the cells, and how Omega-3 oils could possibly help.
Now an exciting stem cell study. An American research group isolated hepatocyte growth factor from mouse mesenchymal stem cells. When they injected this into animals with an MS-like condition, inflammation reduced, neural cells grew, and the myelin sheath regrew over lesions caused by the disease.
Previous work by these researchers has led to a clinical trial where MS patients are being injected with their own stem cells. Now it looks like they may have found the important element for recovery.Watch this space!
Canadian researchers gave injections of either the MS drug interferon beta-1a or a placebo to people who had had signs of possible MS. After 3 years, those who had been on interferon beta-1a were less likely to have progressed to clinically definite MS. This confirms the importance of starting treatment as early as possible.
An oral drug, ONO-4641, is being trialled in the USA. The investigators have found that after 6 months, those on the drug had a reduced number of brain lesions. The medication does have possible side effects, including short-term heart problems, raised liver enzymes, and lowered levels of lymphocytes, an immune system component. The drug does sound promising though, for those unaffected.
Most clinical trials for MS drugs are with people who have relapsing-remitting MS: perhaps natural, since that's the largest group of people with MS. What follows, though, is that in due course the treatments are only approved for use in RRMS, because that's the only group where there's evidence.
So it's good to see a (small) study, of a drug called MIS416, in secondary progressive MS. Although a far bigger study is needed, most of the patients in this trial saw some relief of their symptoms after only 12 weeks. I look forward to seeing further results on this drug.
Finally, some good news for those of us with MS: it seems we may be less likely than average to have heart disease. An American researcher analysed the reasons people with MS had been in hospital, and compared them with people without MS discharged from hospital. The people with MS were less likely to have had heart attacks.
To be honest, I'm not entirely convinced by this. The people with MS were (on average) younger than the others in the analysis, so would be less likely to have cardiovascular problems. And it doesn't seem to take account of the additional risk for people with MS of being hospitalised for infections.
But hey, I'll take all the good news I can!
That's it for this research roundup. See you next time?
The symptoms of MS are caused by damage to the fatty myelin sheath round nerve cells. American researchers found that the immune systems of people with MS were targeting 4 particular lipids (fats). In autopsy, these lipids were depleted in areas of MS damage.
Working with mice with an MS-like illness, they then injected the lipids over several weeks. Disease severity was limited and even reversed. Some tests now show that the mice can take the lipids orally and still improve.
This research is at very early stages, but it holds huge promise for a treatment that can actually repair our damaged myelin.
Omega-3 fatty acids are thought to be helpful for many conditions, but not much work has been done on how they work. American scientists fed three types of fatty acid to mouse macrophages, which they stored as phospholipids. The scientists then stimulated the cells to produce inflammatory responses.They found that Omega-3 inhibited an enzyme called cyclooxygenase (COX), which produces the prostaglandin hormones that spark inflammation.
Inflammation is part of the process of damage to nerve cells that leads to MS symptoms. This research is a big clue as to what could be happening inside the cells, and how Omega-3 oils could possibly help.
Now an exciting stem cell study. An American research group isolated hepatocyte growth factor from mouse mesenchymal stem cells. When they injected this into animals with an MS-like condition, inflammation reduced, neural cells grew, and the myelin sheath regrew over lesions caused by the disease.
Previous work by these researchers has led to a clinical trial where MS patients are being injected with their own stem cells. Now it looks like they may have found the important element for recovery.Watch this space!
Canadian researchers gave injections of either the MS drug interferon beta-1a or a placebo to people who had had signs of possible MS. After 3 years, those who had been on interferon beta-1a were less likely to have progressed to clinically definite MS. This confirms the importance of starting treatment as early as possible.
An oral drug, ONO-4641, is being trialled in the USA. The investigators have found that after 6 months, those on the drug had a reduced number of brain lesions. The medication does have possible side effects, including short-term heart problems, raised liver enzymes, and lowered levels of lymphocytes, an immune system component. The drug does sound promising though, for those unaffected.
Most clinical trials for MS drugs are with people who have relapsing-remitting MS: perhaps natural, since that's the largest group of people with MS. What follows, though, is that in due course the treatments are only approved for use in RRMS, because that's the only group where there's evidence.
So it's good to see a (small) study, of a drug called MIS416, in secondary progressive MS. Although a far bigger study is needed, most of the patients in this trial saw some relief of their symptoms after only 12 weeks. I look forward to seeing further results on this drug.
Finally, some good news for those of us with MS: it seems we may be less likely than average to have heart disease. An American researcher analysed the reasons people with MS had been in hospital, and compared them with people without MS discharged from hospital. The people with MS were less likely to have had heart attacks.
To be honest, I'm not entirely convinced by this. The people with MS were (on average) younger than the others in the analysis, so would be less likely to have cardiovascular problems. And it doesn't seem to take account of the additional risk for people with MS of being hospitalised for infections.
But hey, I'll take all the good news I can!
That's it for this research roundup. See you next time?
Saturday, 23 June 2012
MS Research Roundup June 2012 Part 1
Lots of interesting research to report again this time. It would be a very (very) long post if I did everything together, so I'm going to split it into two. This post will be about risk factors for MS and possible causes, and the second post (to follow shortly) will focus on treatments.
We know that more women than men have MS, and the proportion is increasing. Greek researchers compared the proportions in people living in urban and rural areas in Crete. They found that in rural areas there was less risk of MS generally, and the women:men proportion hadn't risen as it had in the towns.
This is an interesting finding. The researchers found that the urban women were more likely to smoke, drink pasteurised milk, use contraceptives, and various other factors. But so many things could possibly be involved - environmental pollution is one that springs to mind. More research needed!
Research is showing that Vitamin D is linked with a huge number of conditions, including MS. Now it seems it could also be associated with some cases where MS runs in families. British and Canadian researchers have identified a rare gene variant in these families, called CYP27B. People who inherit two copies of this gene develop a genetic form of rickets, a condition caused by vitamin D deficiency. One copy leads to lower than normal levels of vitamin D.
Out of over 3000 unaffected parents of people with MS, 35 carried the gene. In all 35 cases, the person with MS had inherited the gene. The odds against this happening by chance were enormous.
Not only does this finding go some way to explaining how MS can run in families, it also adds to the growing weight of evidence that vitamin D levels and MS are very closely linked.
We also know already that there are links between previous exposure to the Epstein-Barr virus (EBV), which causes glandular fever (mononucleosis), and the risk of developing MS. Spanish researchers discovered that naturally lower levels of vitamin D in winter were linked with lower levels of an immune system component called TLR7. This controls the immune system response to viruses.
The researchers drew no conclusions about the impact of all this on other conditions: but could this be one piece in the puzzle of how the different MS risk factors are linked?
Several projects relating to the CCSVI theory have reported recently. First, two proof of concept studies from the USA, which examined people with MS, people with other conditions, and healthy controls using the Zamboni protocol, using ultrasound and MRV. They found that only a small proportion of people with MS had the venous constrictions said to be characteristic of CCSVI: the proportion for both people with other conditions and healthy people was similar. These studies showed no evidence that narrowed veins are linked with MS.
The European Society of neurosonology and cerebral hemodynamics examined the five criteria for diagnosing CCSVI in the Zamboni ultrasound protocol, along with the studies from which they were derived and the main studies looking at cerebrospinal drainage. Their view is that the criteria are questionable: one is based on data collected in other contexts, two have never been validated, one is technically incorrect, and two are subject to so many external influnces that it's difficult to state whether any "differences" are part of a disease or just normal variation.
As a result of these concerns, the Society strongly discourages venoplasty and/or stenting for CCSVI.
A small study in Canada followed 30 people before and after venoplasty for CCSVI. The researchers found no difference between the patients who had been treated and 10 who had not. Any initial benefits reported tailed off, particularly after about 3 months. By a year, several people's veins had reblocked, but their function was no different from those whose veins had not reblocked.
And a group of Italian reseachers carried out a meta-analysis. They critically analysed the proposed biological basis for CCSVI and reviewed all published studies on CCSVI and its ultrasound methodology. They found no supportive scientific evidence for any part of the theory that CCSVI is linked with MS. They feel that CCSVI may be a stand-alone condition, which should be investigated in its own right - but it's not linked to MS.
People have called me anti-CCSVI. That's not accurate - or wasn't. When I first heard about the idea I was interested. Who wouldn't be? I'd love a cure for my MS. But I knew really rigorous research was needed, to see if Zamboni's results could be repeated. As time has gone on, it's become increasingly apparent that the theory just doesn't hold water. If people want to have their veins Dyno-Rodded, well I suppose that's up to them. But I don't want scarce NHS funds paying for it.
On that (rather controversial) note I'll end Part 1 of this roundup. I'll try to do Part 2, dealing with treatments, tomorrow.
We know that more women than men have MS, and the proportion is increasing. Greek researchers compared the proportions in people living in urban and rural areas in Crete. They found that in rural areas there was less risk of MS generally, and the women:men proportion hadn't risen as it had in the towns.
This is an interesting finding. The researchers found that the urban women were more likely to smoke, drink pasteurised milk, use contraceptives, and various other factors. But so many things could possibly be involved - environmental pollution is one that springs to mind. More research needed!
Research is showing that Vitamin D is linked with a huge number of conditions, including MS. Now it seems it could also be associated with some cases where MS runs in families. British and Canadian researchers have identified a rare gene variant in these families, called CYP27B. People who inherit two copies of this gene develop a genetic form of rickets, a condition caused by vitamin D deficiency. One copy leads to lower than normal levels of vitamin D.
Out of over 3000 unaffected parents of people with MS, 35 carried the gene. In all 35 cases, the person with MS had inherited the gene. The odds against this happening by chance were enormous.
Not only does this finding go some way to explaining how MS can run in families, it also adds to the growing weight of evidence that vitamin D levels and MS are very closely linked.
We also know already that there are links between previous exposure to the Epstein-Barr virus (EBV), which causes glandular fever (mononucleosis), and the risk of developing MS. Spanish researchers discovered that naturally lower levels of vitamin D in winter were linked with lower levels of an immune system component called TLR7. This controls the immune system response to viruses.
The researchers drew no conclusions about the impact of all this on other conditions: but could this be one piece in the puzzle of how the different MS risk factors are linked?
Several projects relating to the CCSVI theory have reported recently. First, two proof of concept studies from the USA, which examined people with MS, people with other conditions, and healthy controls using the Zamboni protocol, using ultrasound and MRV. They found that only a small proportion of people with MS had the venous constrictions said to be characteristic of CCSVI: the proportion for both people with other conditions and healthy people was similar. These studies showed no evidence that narrowed veins are linked with MS.
The European Society of neurosonology and cerebral hemodynamics examined the five criteria for diagnosing CCSVI in the Zamboni ultrasound protocol, along with the studies from which they were derived and the main studies looking at cerebrospinal drainage. Their view is that the criteria are questionable: one is based on data collected in other contexts, two have never been validated, one is technically incorrect, and two are subject to so many external influnces that it's difficult to state whether any "differences" are part of a disease or just normal variation.
As a result of these concerns, the Society strongly discourages venoplasty and/or stenting for CCSVI.
A small study in Canada followed 30 people before and after venoplasty for CCSVI. The researchers found no difference between the patients who had been treated and 10 who had not. Any initial benefits reported tailed off, particularly after about 3 months. By a year, several people's veins had reblocked, but their function was no different from those whose veins had not reblocked.
And a group of Italian reseachers carried out a meta-analysis. They critically analysed the proposed biological basis for CCSVI and reviewed all published studies on CCSVI and its ultrasound methodology. They found no supportive scientific evidence for any part of the theory that CCSVI is linked with MS. They feel that CCSVI may be a stand-alone condition, which should be investigated in its own right - but it's not linked to MS.
People have called me anti-CCSVI. That's not accurate - or wasn't. When I first heard about the idea I was interested. Who wouldn't be? I'd love a cure for my MS. But I knew really rigorous research was needed, to see if Zamboni's results could be repeated. As time has gone on, it's become increasingly apparent that the theory just doesn't hold water. If people want to have their veins Dyno-Rodded, well I suppose that's up to them. But I don't want scarce NHS funds paying for it.
On that (rather controversial) note I'll end Part 1 of this roundup. I'll try to do Part 2, dealing with treatments, tomorrow.
Monday, 9 April 2012
MS Research Roundup April 2012
Some more really interesting MS research projects have reported recently. It's an exciting time!
All of us with MS have probably had MRIs, for diagnosis or to check on progression. But there has always been a paradox: the number of lesions in the brain doesn't correlate with the effects of the disease.
Researchers looked at the number of lesions in different parts of the brain, and found that they could be correlated with specific symptoms. For instance, lesions in the precuneus and precentral gyrus were most predictive of mobility problems.
MRIs have poor resolution, and contrast media such as gadolinium do not necessarily measure what they aim to: they often show fluid rather than inflammation. This research could help MRIs to be used with a new precision, as a truly useful clinical tool.
We already know that many women find their MS symptoms improve during pregnancy. Now Australian researchers have found that even one pregnancy halves a woman's risk of developing MS in the future.For women who have had two or more pregnancies, the risk is a quarter. The researchers suspect this may be the reason the rate of MS in women has inched up over the last few decades, as more and more women have decided to have babies later or not at all.
An interesting study from a group of Belgian researchers. They got nearly 1400 people with relapsing remitting MS about their diet, drinking habits, and whether they smoked. They found that people who consumed alcohol, wine, fish and coffee on a regular basis took 4-7 more years to reach the point where they needed a walking aid than those who never consumed them. They didn't find the same pattern in people with progressive MS. Those who smoked cigarettes needed walking aids earlier than those who didn't.
I'll just have to hope that my intravenous coffee drip offsets my cigarettes then...
Research is continuing on various aspects of the CCSVI theory. The theory states that malformed and blocked veins in the neck lead to the deposition of iron in the brain, which in turn leads to autoimmunity and demyelination of nerve cells.
A group of American researchers tested this theory by tying mice's jugular veins shut. The mice were then observed for 6 months. There were no signs of inflammation or demyelination on CT or MRI, and no clinical change. This result strongly suggests that CCSVI is not responsible for demyelination, and therefore not responsible for MS.
We've recently seen the approval of the first oral drug for MS, Gilenya. The next to come into clinical use is likely to be BG-12, which has had extremely good results in trials. When taken three times a day, it halved the relapse rate compared to a placebo tablet.
Now the manufacturers, Biogen Idec, have applied to the FDA for approval for BG-12. They're hoping for priority processing which will shave some time off the process: if they don't get it, BG-12 should be released in the USA by the end of 2012 or early in 2013. Hopefully it'll be introduced in Europe soon afterwards, and so on around the globe.
The next study is of particular interest to me, as I've recently had this treatment myself. (Watch this space - I'm planning to post about it.)
BOTOX® is already approved in several countries including the UK and USA for the treatment of overactive bladder resulting from neurological problems such as MS and spinal cord injury. It works by relaxing the bladder muscles that were previously going into spasm, causing urgency, frequency, and possible incontinence.
Now two Phase 3 studies have shown positive results for BOTOX® for idiopathic (cause not known) overactive bladder. These results are just further confirmation of the usefulness of BOTOX® in treating overactive bladder.
Many people with MS fall: falls can result in injury, and the fear of falling can result in severely restricted mobility. American researchers asked 575 people with MS about their level of disability, how often they fell, and how afraid they were of falling. Nearly two thirds were concerned about falling, and over two thirds restricted their activities because of fear of falling. People with moderate mobility problems had the highest number of falls, and those with the worst mobility (ie non-walkers) had the fewest.
What can we do with this information? I suppose it's a question of awareness.If you know you're in the group most at risk of falls - the group with moderate mobility problems - you need to take particular care not to lean too far over, not to turn your body without moving your feet, and so on.
Researchers in Germany asked several hundred people who felt severely affected by their MS whether they wanted their doctors to talk to them about their disease progression, and about end-of-life issues.Three-quarters wanted to discuss their disease progression, but less than half were interested in talking about end-of-life issues like whether they would want to go on a life support machine or whether they would want to be resuscitated should the need arise.
These are important but very sensitive topics. Doctors must show great sensitivity when raising them.
So that's it for this research roundup.See you next time!
All of us with MS have probably had MRIs, for diagnosis or to check on progression. But there has always been a paradox: the number of lesions in the brain doesn't correlate with the effects of the disease.
Researchers looked at the number of lesions in different parts of the brain, and found that they could be correlated with specific symptoms. For instance, lesions in the precuneus and precentral gyrus were most predictive of mobility problems.
MRIs have poor resolution, and contrast media such as gadolinium do not necessarily measure what they aim to: they often show fluid rather than inflammation. This research could help MRIs to be used with a new precision, as a truly useful clinical tool.
We already know that many women find their MS symptoms improve during pregnancy. Now Australian researchers have found that even one pregnancy halves a woman's risk of developing MS in the future.For women who have had two or more pregnancies, the risk is a quarter. The researchers suspect this may be the reason the rate of MS in women has inched up over the last few decades, as more and more women have decided to have babies later or not at all.
An interesting study from a group of Belgian researchers. They got nearly 1400 people with relapsing remitting MS about their diet, drinking habits, and whether they smoked. They found that people who consumed alcohol, wine, fish and coffee on a regular basis took 4-7 more years to reach the point where they needed a walking aid than those who never consumed them. They didn't find the same pattern in people with progressive MS. Those who smoked cigarettes needed walking aids earlier than those who didn't.
I'll just have to hope that my intravenous coffee drip offsets my cigarettes then...
Research is continuing on various aspects of the CCSVI theory. The theory states that malformed and blocked veins in the neck lead to the deposition of iron in the brain, which in turn leads to autoimmunity and demyelination of nerve cells.
A group of American researchers tested this theory by tying mice's jugular veins shut. The mice were then observed for 6 months. There were no signs of inflammation or demyelination on CT or MRI, and no clinical change. This result strongly suggests that CCSVI is not responsible for demyelination, and therefore not responsible for MS.
We've recently seen the approval of the first oral drug for MS, Gilenya. The next to come into clinical use is likely to be BG-12, which has had extremely good results in trials. When taken three times a day, it halved the relapse rate compared to a placebo tablet.
Now the manufacturers, Biogen Idec, have applied to the FDA for approval for BG-12. They're hoping for priority processing which will shave some time off the process: if they don't get it, BG-12 should be released in the USA by the end of 2012 or early in 2013. Hopefully it'll be introduced in Europe soon afterwards, and so on around the globe.
The next study is of particular interest to me, as I've recently had this treatment myself. (Watch this space - I'm planning to post about it.)
BOTOX® is already approved in several countries including the UK and USA for the treatment of overactive bladder resulting from neurological problems such as MS and spinal cord injury. It works by relaxing the bladder muscles that were previously going into spasm, causing urgency, frequency, and possible incontinence.
Now two Phase 3 studies have shown positive results for BOTOX® for idiopathic (cause not known) overactive bladder. These results are just further confirmation of the usefulness of BOTOX® in treating overactive bladder.
Many people with MS fall: falls can result in injury, and the fear of falling can result in severely restricted mobility. American researchers asked 575 people with MS about their level of disability, how often they fell, and how afraid they were of falling. Nearly two thirds were concerned about falling, and over two thirds restricted their activities because of fear of falling. People with moderate mobility problems had the highest number of falls, and those with the worst mobility (ie non-walkers) had the fewest.
What can we do with this information? I suppose it's a question of awareness.If you know you're in the group most at risk of falls - the group with moderate mobility problems - you need to take particular care not to lean too far over, not to turn your body without moving your feet, and so on.
Researchers in Germany asked several hundred people who felt severely affected by their MS whether they wanted their doctors to talk to them about their disease progression, and about end-of-life issues.Three-quarters wanted to discuss their disease progression, but less than half were interested in talking about end-of-life issues like whether they would want to go on a life support machine or whether they would want to be resuscitated should the need arise.
These are important but very sensitive topics. Doctors must show great sensitivity when raising them.
So that's it for this research roundup.See you next time!
Saturday, 25 February 2012
Life's a trial
When I'm doing research roundups, I quite often talk about clinical trials being "Phase 2" or "Phase 3". But what do these terms mean? The development of a new drug or other intervention is a long-term process. commonly taking 12 or more years before being available to patients. The regulatory process adds another hefty chunk of time.What's happening for it all to take so long?
Research begins in the laboratory, where potential treatments are tested on animals: for instance, there is a strain of mice which have a condition very like MS. Testing drugs on them gives a reasonable idea of whether they're likely to help people with MS. Around 1000 potential drugs are tested for each one that makes it to clinical trials.
The use of animals in drug trials is a whole other question, one I might discuss in a future blog post. If the drug is helpful for the animals (and how that's worked out is again a subject for a future blog post), the researchers will look for more funding to test it out on people, in clinical trials. The clinical trial process has four phases.
Phase 0 Very low doses of the drug are given to 10-15 people to see whether the drug does what was expected in humans. Tests are carried out to check what the drug does to the body, and what the body does to the drug.
Phase I The drug is tested on usually 20-100 healthy volunteers, to check that it is safe. These volunteers are normally paid, as they won't get any health benefit from participating, and are taking the risk of being given an untested drug. Some of you may remember news coverage of a Phase 1 trial in 2006 where 6 healthy volunteers became violently ill after taking a new drug.
Phase II Designed to assess how well the drug works, and what is the best dosage. Between 100 and 300 volunteer patients take either the drug or something else - the existing treatment if there is one, or a placebo.
Phase III The drug is tested on a large number of patients over a number of sites. This phase aims to be the definitive assessment of how effective the drug is, compared with any current treatments. Sometimes a manufacturer wants to prove that their drug works for other patients or other conditions than those originally established. In that case the Phase 2 or 3 trial would be the first stage.
Phase IV This is the period of surveillance once the drug is on the market. Safety continues to be checked, and technical support is available.
Clinical trials can take a long time to run. It can be difficult to recruit the number of people needed, particularly in Phase III. For many long-term conditions, it can take several months to see any effect from the drug. I recently participated in a Phase II trial for a full year.
Most Phase III trials (and some Phase II) are randomised, double-blind and controlled.
Their decisions are based on cost-effectiveness, potentially leading to some controversial outcomes. Recently they've refused funding to the new MS drug Gilenya, and there have been several decisions where funding has been refused for expensive cancer drugs which were likely to give only a few more months of life.
In some cases, local Primary Care Trusts still have to agree to fund the treatment. There have been problems recently with Sativex, which is licensed for use in MS spasticity if other treatments don't help. Many PCTs are refusing to fund it. The MS Society is campaigning on this: if you have funding for Sativex refused, they provide advice on what steps to take.
You're most likely to find out about trials through your consultant. If you'd be interested in participating in a research project (without commiting yourself to anything!) let them know.
It can't be denied that there are some risks involved, as there are with any treatment. But I found trial participation interesting, and if the drug concerned goes on to be approved I'll feel quite proud: I was part of that!
Research begins in the laboratory, where potential treatments are tested on animals: for instance, there is a strain of mice which have a condition very like MS. Testing drugs on them gives a reasonable idea of whether they're likely to help people with MS. Around 1000 potential drugs are tested for each one that makes it to clinical trials.
The use of animals in drug trials is a whole other question, one I might discuss in a future blog post. If the drug is helpful for the animals (and how that's worked out is again a subject for a future blog post), the researchers will look for more funding to test it out on people, in clinical trials. The clinical trial process has four phases.
Phase 0 Very low doses of the drug are given to 10-15 people to see whether the drug does what was expected in humans. Tests are carried out to check what the drug does to the body, and what the body does to the drug.
Phase I The drug is tested on usually 20-100 healthy volunteers, to check that it is safe. These volunteers are normally paid, as they won't get any health benefit from participating, and are taking the risk of being given an untested drug. Some of you may remember news coverage of a Phase 1 trial in 2006 where 6 healthy volunteers became violently ill after taking a new drug.
Phase II Designed to assess how well the drug works, and what is the best dosage. Between 100 and 300 volunteer patients take either the drug or something else - the existing treatment if there is one, or a placebo.
Phase III The drug is tested on a large number of patients over a number of sites. This phase aims to be the definitive assessment of how effective the drug is, compared with any current treatments. Sometimes a manufacturer wants to prove that their drug works for other patients or other conditions than those originally established. In that case the Phase 2 or 3 trial would be the first stage.
Phase IV This is the period of surveillance once the drug is on the market. Safety continues to be checked, and technical support is available.
Clinical trials can take a long time to run. It can be difficult to recruit the number of people needed, particularly in Phase III. For many long-term conditions, it can take several months to see any effect from the drug. I recently participated in a Phase II trial for a full year.
Most Phase III trials (and some Phase II) are randomised, double-blind and controlled.
- Randomised means that participants are randomly assigned to the treatment or placebo groups
- Double-blind means that neither the participant nor the researchers know whether they're receiving the treatment or the placebo. It's important that the researchers don't know, as they might subconsciously behave differently to people in the two groups.
- Controlled means that one group receives a placebo (or the existing treatment, if there is one).This means that the effect of the new drug can be isolated. Is it better than the existing treatment? If so, how much better?
- Some trials are designed to cross-over, This means that halfway through the trial period, the participants swap over to receiving the other treatment. Those who were gettng the active drug will change onto the placebo, and vice versa.
Their decisions are based on cost-effectiveness, potentially leading to some controversial outcomes. Recently they've refused funding to the new MS drug Gilenya, and there have been several decisions where funding has been refused for expensive cancer drugs which were likely to give only a few more months of life.
In some cases, local Primary Care Trusts still have to agree to fund the treatment. There have been problems recently with Sativex, which is licensed for use in MS spasticity if other treatments don't help. Many PCTs are refusing to fund it. The MS Society is campaigning on this: if you have funding for Sativex refused, they provide advice on what steps to take.
You're most likely to find out about trials through your consultant. If you'd be interested in participating in a research project (without commiting yourself to anything!) let them know.
It can't be denied that there are some risks involved, as there are with any treatment. But I found trial participation interesting, and if the drug concerned goes on to be approved I'll feel quite proud: I was part of that!
Thursday, 23 February 2012
MS research roundup February 2012
With apologies for the long gap since the last one - here, finally, is my latest research roundup. And there's some real goodies this time!
MS is conventionally seen as an auto-immune condition. For some reason, not yet fully understood, the body's immune system begins to attack the myelin sheath surrounding the nerves. This has recently been challenged by the CCSVI theory, which states that MS is caused by blockages in the veins of the neck leading to back-up of blood and consequent deposition of iron in the brain.
Now an American researcher has developed a new theory. She believes that MS is a metabolic disease, caused by the body having problems dealing with fats in the diet. The rise in the number of cases over the last few decades is due to us, as a population, moving to a diet high in carbohydrates and saturated fats. Oxidised LDL cholesterol accumulates on nerve cells and triggers an immune response, leading to scarring of the cells. Men and women metabolise fats differently, which could account for the higher prevalence of MS in women.
It's an interesting theory, but obviously needs much research done to see if it holds water. Watch this space..
I've talked about vitamin D several times before: there's a lot of research going on in the field, and there seem to be many links between vitamin D and MS. Vitamin D is created in the body following exposure to sunlight, so people tend to be more at risk of deficiencies the further they are from the equator.
An international group of researchers are now suggesting that vitamin D should be added to foods like milk and fruit juice in Scotland, which has some of the highest levels of MS in the world. The Scottish Parliament is unconvinced. Food supplementation already exists: iodine is added to table salt to protect against goitre. Should vitamin D be added to the list?
As many of us know all too well, MS can be difficult and time-consuming to diagnose. This can cause distressing uncertainty, as well as delays in commencing treatments. Israeli researchers have developed an electronic "nose" which can diagnose MS, as well as some cancers, from a person's breath. They identified organic compounds in the breath that are a sign of MS, then produced sensors to detect them. They hope that their device will allow MS to be diagnosed at an early stage and without using invasive techniques like lumbar punctures. Still early stages for this research, but a very exciting development!
On to treatments. At least 80% of people using interferon-b eventually develop antibodies to it, meaning treatment has to be stopped. Instead, German researchers tried injecting mice with an MS-like disease with a type of RNA which stimulates the body to produce its own interferon-b. The results were excellent. This could prove to be an excellent way round the antibody problem.
As people with MS age, the rate of remyelination of nerve fibres slows, resulting in the loss of more nerves. A research group in the UK have managed to reverse this decline in mice by exposing them to stem cells from young mice. Stem cells show great promise in remyelination, and this is a great discovery.
Finally, research on treatments for MS symptoms.
The MS International Federation have released the results of a survey on fatigue. 86% of those who responded said that fatigue was one of their main symptoms, and 46% that fatigue has a high impact on their lives. For those of us with MS fatigue, this will come as little surprise. Interesting data, showing how important it is for healthcare providers to recognise the problem of fatigue. Important to remember, though, that those who responded had chosen to participate in a survey on fatigue, and might therefore be those for whom it's an important consideration. People unaffected by fatigue might not have been interested in participating.
An Israeli research group did a trial involving three groups of patients. One group received electrical stimulation of the left prefrontal cortex, one of the motor cortex, and the final, control, group received a sham stimulation. After 18 treatments, the group receiving stimulation in the motor cortex had less fatigue and less depression. There was a tendency towards less fatigue in the prefrontal cortex group. The control group had no signnificant improvement. Further research is planned to investigate the effect of the stimulation on metabolic and neural activity.
Many of us with MS also experience cognitive problems - short term memory loss, lack of concentration, and so on. Italian researchers used computer-based rehabilitation programmes to help with attention and information processing. One programme, for instance, is a train driving simulation: you have to observe the control panel of the train and the surrounding countryside while encountering increasingly difficult distractions. After a 12-week programme of treatment, patients had improved attention and information processing skills, although no changes were seen on MRI. It seems training can help, but it's no cure.
I hope you agree that there were some really promising bits of research this time. Let's see what happens next!
MS is conventionally seen as an auto-immune condition. For some reason, not yet fully understood, the body's immune system begins to attack the myelin sheath surrounding the nerves. This has recently been challenged by the CCSVI theory, which states that MS is caused by blockages in the veins of the neck leading to back-up of blood and consequent deposition of iron in the brain.
Now an American researcher has developed a new theory. She believes that MS is a metabolic disease, caused by the body having problems dealing with fats in the diet. The rise in the number of cases over the last few decades is due to us, as a population, moving to a diet high in carbohydrates and saturated fats. Oxidised LDL cholesterol accumulates on nerve cells and triggers an immune response, leading to scarring of the cells. Men and women metabolise fats differently, which could account for the higher prevalence of MS in women.
It's an interesting theory, but obviously needs much research done to see if it holds water. Watch this space..
I've talked about vitamin D several times before: there's a lot of research going on in the field, and there seem to be many links between vitamin D and MS. Vitamin D is created in the body following exposure to sunlight, so people tend to be more at risk of deficiencies the further they are from the equator.
An international group of researchers are now suggesting that vitamin D should be added to foods like milk and fruit juice in Scotland, which has some of the highest levels of MS in the world. The Scottish Parliament is unconvinced. Food supplementation already exists: iodine is added to table salt to protect against goitre. Should vitamin D be added to the list?
As many of us know all too well, MS can be difficult and time-consuming to diagnose. This can cause distressing uncertainty, as well as delays in commencing treatments. Israeli researchers have developed an electronic "nose" which can diagnose MS, as well as some cancers, from a person's breath. They identified organic compounds in the breath that are a sign of MS, then produced sensors to detect them. They hope that their device will allow MS to be diagnosed at an early stage and without using invasive techniques like lumbar punctures. Still early stages for this research, but a very exciting development!
On to treatments. At least 80% of people using interferon-b eventually develop antibodies to it, meaning treatment has to be stopped. Instead, German researchers tried injecting mice with an MS-like disease with a type of RNA which stimulates the body to produce its own interferon-b. The results were excellent. This could prove to be an excellent way round the antibody problem.
As people with MS age, the rate of remyelination of nerve fibres slows, resulting in the loss of more nerves. A research group in the UK have managed to reverse this decline in mice by exposing them to stem cells from young mice. Stem cells show great promise in remyelination, and this is a great discovery.
Finally, research on treatments for MS symptoms.
The MS International Federation have released the results of a survey on fatigue. 86% of those who responded said that fatigue was one of their main symptoms, and 46% that fatigue has a high impact on their lives. For those of us with MS fatigue, this will come as little surprise. Interesting data, showing how important it is for healthcare providers to recognise the problem of fatigue. Important to remember, though, that those who responded had chosen to participate in a survey on fatigue, and might therefore be those for whom it's an important consideration. People unaffected by fatigue might not have been interested in participating.
An Israeli research group did a trial involving three groups of patients. One group received electrical stimulation of the left prefrontal cortex, one of the motor cortex, and the final, control, group received a sham stimulation. After 18 treatments, the group receiving stimulation in the motor cortex had less fatigue and less depression. There was a tendency towards less fatigue in the prefrontal cortex group. The control group had no signnificant improvement. Further research is planned to investigate the effect of the stimulation on metabolic and neural activity.
Many of us with MS also experience cognitive problems - short term memory loss, lack of concentration, and so on. Italian researchers used computer-based rehabilitation programmes to help with attention and information processing. One programme, for instance, is a train driving simulation: you have to observe the control panel of the train and the surrounding countryside while encountering increasingly difficult distractions. After a 12-week programme of treatment, patients had improved attention and information processing skills, although no changes were seen on MRI. It seems training can help, but it's no cure.
I hope you agree that there were some really promising bits of research this time. Let's see what happens next!
Wednesday, 14 December 2011
Share your experience!
I'd like to introduce you to a great website. Health Talk Online is run by the Health Experience Research Group at the University of Oxford.
The Research Group collects personal stories of health, illness and disability. As well as analysing these to use in academic publications, it makes the stories available on the website, as video or audio clips, so others with the same conditions can find support and information. There's a list of the conditions they've covered so far here.
At the moment, the researchers are looking for people with acquired conditions who live in London. Would you be interested in helping them? Contact details are here.They also want to speak to carers of people with MS, anywhere in the UK. Is that you, or do you know someone who is? Contact details are on the same page.
I know all and any help will be very gratefully received.
The Research Group collects personal stories of health, illness and disability. As well as analysing these to use in academic publications, it makes the stories available on the website, as video or audio clips, so others with the same conditions can find support and information. There's a list of the conditions they've covered so far here.
At the moment, the researchers are looking for people with acquired conditions who live in London. Would you be interested in helping them? Contact details are here.They also want to speak to carers of people with MS, anywhere in the UK. Is that you, or do you know someone who is? Contact details are on the same page.
I know all and any help will be very gratefully received.
Friday, 9 December 2011
Research roundup December 2011: Blogger ate (most of) my post
This was going to be a lovely long, informative research roundup, full of interesting and groundbreaking stuff to take you through to the New Year.
Sadly, Blogger (the software on this site) decided to eat all my carefully saved links. And what with my terrible MS memory, I can only remember two of them!
Not to be daunted, I'm going to tell you about those two. I'm really sorry though. Hopefully I'll remember some of the others, and can add them to other roundups in the future.
I've often spoken before about vitamin D and MS. It's a very important area of research at the moment. Now a group of British and Canadian researchers have discovered a rare gene that seems to be linked to MS. The gene is called CYP27B1: a mutation in it affects a key enzyme, leading people with the variant to have lower levels of vitamin D.
They identified the mutation in CYP27B1 as being of interest, from looking at 43 people who came from families with 4 or more individuals affected by MS. Then they looked at over 3,000 unaffected parents of someone with MS. 35 of them carried one copy of the mutated version of CYP27B1 along with a copy of the normal version. In all 35 of these cases, the person with MS inherited the mutated version.
The likelihood of the gene's transmission being unconnected with MS are billions to one, the researchers claim. The very strong implication is that in these particular MS cases, low levels of vitamin D are directly connected to the disease.
It's always been thought that MS begins by attacking myelin in the inner layers of the brain, the white matter. Now American researchers have found that in fact the disease seems to move from the outer, cortical layers of the brain.
The next step for their research is to study these cortical lesions to identify new molecular targets for treatment, and to develop imaging techniques so that these lesions can be shown clearly.
And, well, that's it for December's roundup. Apologies again for the lack of content!
Sadly, Blogger (the software on this site) decided to eat all my carefully saved links. And what with my terrible MS memory, I can only remember two of them!
Not to be daunted, I'm going to tell you about those two. I'm really sorry though. Hopefully I'll remember some of the others, and can add them to other roundups in the future.
I've often spoken before about vitamin D and MS. It's a very important area of research at the moment. Now a group of British and Canadian researchers have discovered a rare gene that seems to be linked to MS. The gene is called CYP27B1: a mutation in it affects a key enzyme, leading people with the variant to have lower levels of vitamin D.
They identified the mutation in CYP27B1 as being of interest, from looking at 43 people who came from families with 4 or more individuals affected by MS. Then they looked at over 3,000 unaffected parents of someone with MS. 35 of them carried one copy of the mutated version of CYP27B1 along with a copy of the normal version. In all 35 of these cases, the person with MS inherited the mutated version.
The likelihood of the gene's transmission being unconnected with MS are billions to one, the researchers claim. The very strong implication is that in these particular MS cases, low levels of vitamin D are directly connected to the disease.
It's always been thought that MS begins by attacking myelin in the inner layers of the brain, the white matter. Now American researchers have found that in fact the disease seems to move from the outer, cortical layers of the brain.
The next step for their research is to study these cortical lesions to identify new molecular targets for treatment, and to develop imaging techniques so that these lesions can be shown clearly.
And, well, that's it for December's roundup. Apologies again for the lack of content!
Thursday, 6 October 2011
MS Research Roundup October 2011
There's been some very interesting research announced this month. Some of it is pretty technical, but once I've worked out what it means myself, I'll do my best to translate it!
American and Canadian researchers have developed an imaging technique using lasers that allows them to see the amount of damage the myelin sheaths around nerves have sustained. Previously, such measurements could only be obtained by removing the nerve and slicing it into layers - which was only possible once the patient was dead! So far, this research has only been carried out on animals, but it could in the future be used as a diagnostic tool for conditions like MS.
The body has developed what is generally a very effective barrier between the blood and the brain, to keep anything potentially harmful from moving between the two. While this is generally very useful, it can pose a problem with getting drugs into the brain to treat conditions like MS, Alzheimer's disease, and cancers of the central nervous system.
Now a group of American researchers have found that a molecule called adenosine can help large molecules enter the brain. An existing drug called Lexiscan, which is based on adenosine and is used in heart imaging for very ill patients, briefly opens the blood-brain barrier. In MS, confusingly, research is also focusing on how to tighten the barrier, to stop destructive immune cells getting in to the brain and causing damage.
There's been a lot of research, and talk in the MS community, about the possible beneficial effects of taking fish oils for people with MS. Researchers from America looked at the effects of taking omega-3 fatty acid. They found that it had a significant impact on a molecule called matrix metalloproteinase-9 (MMP-9), which is associated with disruption of the blood-brain barrier, and immune system T cells getting into the brain. Their results suggest that taking Omega-3 supplements may benefit people with MS: they don't suggest amounts. That (as ever) will be for further research.
Another group of American researchers looked at how the body metabolises fatty acids. The speed of metabolism is controlled by an enzyme called carnitine palmitoyltransferase 1 (CPT-1). The researchers controlled the amount of CPT-1 in the animals they were working with, and found a reduction in disease severity as well as less inflammation and demyelination.This research is a long way from any clinical use, but it sounds a potentially useful therapeutic target for MS.
Certain steroids in the brain (neurosteroids) have a protective function. Canadian and Iranian researchers discovered that brain tissue from people with MS had much lower levels of neurosteroids than tissue from people without, particularly a neurosteroid called allopregnanolone.This was due to the action of a specific molecule called micro-RNA (miRNA).
They then treated mice with a disease similar to MS with injections of allopregnanolone and found that they maintained a better protective myelin coating on their spinal cords than mice receiving the placebo.They also had significantly reduced disease severity compared both with the mice receiving the placebo and their own symptoms before treatment.
Next, two treatment stories relevant to particular countries. The first North American stem cell trial for MS has been approved: as a Phase 1 trial it will assess the feasibility and safety of using the body's own stem cells to treat MS.
The procedure will consist of harvesting the patient's mesenchymal stem cells, culturing them in a laboratory, and then injecting them intravenously back into the patient. Mesenchymal stem cells have a wide range of effects, varying from lessening immune activity to encouraging tissue repair. Study participants will be closely monitored for six months after the procedure. If it's shown to be safe and feasible, an application may be made for a larger, controlled trial, including the use of a placebo procedure.
Fampridine (Fampyra) has just been launched in the UK. I did a separate post about this drug, which aids walking in some people with MS.
Finally for this month, an Italian research group have been researching treatments for fatigue in MS. They used neurocognitive rehabilitation techniques. The information I have about the research doesn't say what specific techniques they used, but neurocognitive rehabilitation can include things like occupational therapy and hand/eye coordination exercises.
The researchers found that both immediately after 5 weeks treatment and 6 months later less fatigue was reported. There was no difference in physical disability. They suggest that neurocognitive rehabilitation could be a useful strategy for treating people with MS.
That's the roundup rounded up for this month. What will the next month bring? Only time will tell!
American and Canadian researchers have developed an imaging technique using lasers that allows them to see the amount of damage the myelin sheaths around nerves have sustained. Previously, such measurements could only be obtained by removing the nerve and slicing it into layers - which was only possible once the patient was dead! So far, this research has only been carried out on animals, but it could in the future be used as a diagnostic tool for conditions like MS.
The body has developed what is generally a very effective barrier between the blood and the brain, to keep anything potentially harmful from moving between the two. While this is generally very useful, it can pose a problem with getting drugs into the brain to treat conditions like MS, Alzheimer's disease, and cancers of the central nervous system.
Now a group of American researchers have found that a molecule called adenosine can help large molecules enter the brain. An existing drug called Lexiscan, which is based on adenosine and is used in heart imaging for very ill patients, briefly opens the blood-brain barrier. In MS, confusingly, research is also focusing on how to tighten the barrier, to stop destructive immune cells getting in to the brain and causing damage.
There's been a lot of research, and talk in the MS community, about the possible beneficial effects of taking fish oils for people with MS. Researchers from America looked at the effects of taking omega-3 fatty acid. They found that it had a significant impact on a molecule called matrix metalloproteinase-9 (MMP-9), which is associated with disruption of the blood-brain barrier, and immune system T cells getting into the brain. Their results suggest that taking Omega-3 supplements may benefit people with MS: they don't suggest amounts. That (as ever) will be for further research.
Another group of American researchers looked at how the body metabolises fatty acids. The speed of metabolism is controlled by an enzyme called carnitine palmitoyltransferase 1 (CPT-1). The researchers controlled the amount of CPT-1 in the animals they were working with, and found a reduction in disease severity as well as less inflammation and demyelination.This research is a long way from any clinical use, but it sounds a potentially useful therapeutic target for MS.
Certain steroids in the brain (neurosteroids) have a protective function. Canadian and Iranian researchers discovered that brain tissue from people with MS had much lower levels of neurosteroids than tissue from people without, particularly a neurosteroid called allopregnanolone.This was due to the action of a specific molecule called micro-RNA (miRNA).
They then treated mice with a disease similar to MS with injections of allopregnanolone and found that they maintained a better protective myelin coating on their spinal cords than mice receiving the placebo.They also had significantly reduced disease severity compared both with the mice receiving the placebo and their own symptoms before treatment.
Next, two treatment stories relevant to particular countries. The first North American stem cell trial for MS has been approved: as a Phase 1 trial it will assess the feasibility and safety of using the body's own stem cells to treat MS.
The procedure will consist of harvesting the patient's mesenchymal stem cells, culturing them in a laboratory, and then injecting them intravenously back into the patient. Mesenchymal stem cells have a wide range of effects, varying from lessening immune activity to encouraging tissue repair. Study participants will be closely monitored for six months after the procedure. If it's shown to be safe and feasible, an application may be made for a larger, controlled trial, including the use of a placebo procedure.
Fampridine (Fampyra) has just been launched in the UK. I did a separate post about this drug, which aids walking in some people with MS.
Finally for this month, an Italian research group have been researching treatments for fatigue in MS. They used neurocognitive rehabilitation techniques. The information I have about the research doesn't say what specific techniques they used, but neurocognitive rehabilitation can include things like occupational therapy and hand/eye coordination exercises.
The researchers found that both immediately after 5 weeks treatment and 6 months later less fatigue was reported. There was no difference in physical disability. They suggest that neurocognitive rehabilitation could be a useful strategy for treating people with MS.
That's the roundup rounded up for this month. What will the next month bring? Only time will tell!
Saturday, 10 September 2011
MS Research Roundup September 2011
Again, a short research review this month. My apologies.
First, an extremely interesting and important genetic study, that has received a lot of media attention. Researchers looked at more than 10,000 people with MS from 15 countries, as well as more than 17,000 healthy controls. We already knew about more than 20 gene differerences that may contribute to people getting MS. The large numbers involved in this study allowed them to find another 29 genetic variants that could contribute.
About half of all the gene variants known to be involved affect the immune system, and one-third have been implicated in other auto-immune diseases, such as Crohn's disease, coeliac disease, rheumatoid arthritis, lupus, and type 1 diabetes.Knowing which genes are involved in MS could potentially help new treatments be developed, including the possibility that treatments already in use for one auto-immune condition could be used for another.
I've mentioned the common herpes virus HHV-6 before. It causes the childhood infection roseola, marked by a chest rash and high fever. However very little is known about it, including how it manages to reach the brain to be implicated in causing conditions like MS, encephalitis and a form of epilepsy.
These American researchers found high levels of HHV-6 in the olfactory bulb (a part of the brain associated with smelling) and in nasal mucus, in over half of their samples. They also discovered that specialised cells connecting the nose to the brain are susceptible to infection by the virus. As ever, more research needed. It could be that infection entering the brain via the nose causes a different disease result from infection entering by another source. This is a really interesting study though.
It's well-known that MS, as well as other auto-immune diseases like lupus and rheumatoid arthritis, are considerably more likely to attack women than men, but it's never been clear why. Now American researchers have discovered a new type of immune system B cell: these make antibodies, which go on to bind to and attack the body's own tissues.
The researchers found higher levels of these cells in elderly female mice, young and old mice prone to auto-immune disease, and humans with auto-immune diseases. They are now seeking a way to treat these diseases by blocking production of the cells.
A very big MS-related story at the moment is vitamin D exposure. A group of American researchers compared vitamin D exposure between childhood (whether the individual had been given cod liver oil) and disease onset (sun exposure in the previous winter) with how rapidly they were progressing.
They found a close link between a low level of vitamin D exposure before disease onset and slower disease progression. They were working only with people with progressive MS, and with quite a small group, so research is needed with a larger group and with people with the relapsing remitting form as well to see if the same results are seen. A really worthwhile and interesting study though.
Daclizumab (Zenapax) is a monoclonal antibody, currently going through trials. In this recent global trial, where daclizumab was injected every four weeks, it reduced relapse rates by around half compared to taking a placebo. There was however an increased risk of infections. Daclizumab is now in phase 3 trials, where it's being compared with beta interferon 1a.
Finally, news on two treatments for MS symptoms. First, the Irish Medicines Board has given approval to the use of Botox for urinary incontinence for MS following the biggest ever clinical trial of Botox for neurogenic overactivity of the detrusor muscle. The study demonstrated that treatment was effective within a couple of weeks and lasted for 8-10 months.
Botox is already in use in the UK, and this large study just confirms its utility. It's an important step towards gaining licences in those countries where it's not yet used.
Many of us with MS, diabetes or shingles know the terrible, ongoing problem caused by nerve pain. Current drugs may not help at all. Now British researchers have discovered that a gene called HCN2 is involved in causing the excessive "firing" of pain-sensitive nerves.
In experiments with mice genetically modified to have the gene removed, disabling the gene was found to relieve chronic and inflammatory pain, but without reducing reactions to acute pain, the type caused by a sudden injury. This is crucial: responses to injuries can be vital to our survival. The researchers hope that they'll be able to develop a drug to block the protein produced by HCN2, so the same effect can be seen in humans.
That concludes this month's research review. Again, apologies for the limited number of items covered, but I hope you'll agree there are some really interesting studies in there.
See you next month!
First, an extremely interesting and important genetic study, that has received a lot of media attention. Researchers looked at more than 10,000 people with MS from 15 countries, as well as more than 17,000 healthy controls. We already knew about more than 20 gene differerences that may contribute to people getting MS. The large numbers involved in this study allowed them to find another 29 genetic variants that could contribute.
About half of all the gene variants known to be involved affect the immune system, and one-third have been implicated in other auto-immune diseases, such as Crohn's disease, coeliac disease, rheumatoid arthritis, lupus, and type 1 diabetes.Knowing which genes are involved in MS could potentially help new treatments be developed, including the possibility that treatments already in use for one auto-immune condition could be used for another.
I've mentioned the common herpes virus HHV-6 before. It causes the childhood infection roseola, marked by a chest rash and high fever. However very little is known about it, including how it manages to reach the brain to be implicated in causing conditions like MS, encephalitis and a form of epilepsy.
These American researchers found high levels of HHV-6 in the olfactory bulb (a part of the brain associated with smelling) and in nasal mucus, in over half of their samples. They also discovered that specialised cells connecting the nose to the brain are susceptible to infection by the virus. As ever, more research needed. It could be that infection entering the brain via the nose causes a different disease result from infection entering by another source. This is a really interesting study though.
It's well-known that MS, as well as other auto-immune diseases like lupus and rheumatoid arthritis, are considerably more likely to attack women than men, but it's never been clear why. Now American researchers have discovered a new type of immune system B cell: these make antibodies, which go on to bind to and attack the body's own tissues.
The researchers found higher levels of these cells in elderly female mice, young and old mice prone to auto-immune disease, and humans with auto-immune diseases. They are now seeking a way to treat these diseases by blocking production of the cells.
A very big MS-related story at the moment is vitamin D exposure. A group of American researchers compared vitamin D exposure between childhood (whether the individual had been given cod liver oil) and disease onset (sun exposure in the previous winter) with how rapidly they were progressing.
They found a close link between a low level of vitamin D exposure before disease onset and slower disease progression. They were working only with people with progressive MS, and with quite a small group, so research is needed with a larger group and with people with the relapsing remitting form as well to see if the same results are seen. A really worthwhile and interesting study though.
Daclizumab (Zenapax) is a monoclonal antibody, currently going through trials. In this recent global trial, where daclizumab was injected every four weeks, it reduced relapse rates by around half compared to taking a placebo. There was however an increased risk of infections. Daclizumab is now in phase 3 trials, where it's being compared with beta interferon 1a.
Finally, news on two treatments for MS symptoms. First, the Irish Medicines Board has given approval to the use of Botox for urinary incontinence for MS following the biggest ever clinical trial of Botox for neurogenic overactivity of the detrusor muscle. The study demonstrated that treatment was effective within a couple of weeks and lasted for 8-10 months.
Botox is already in use in the UK, and this large study just confirms its utility. It's an important step towards gaining licences in those countries where it's not yet used.
Many of us with MS, diabetes or shingles know the terrible, ongoing problem caused by nerve pain. Current drugs may not help at all. Now British researchers have discovered that a gene called HCN2 is involved in causing the excessive "firing" of pain-sensitive nerves.
In experiments with mice genetically modified to have the gene removed, disabling the gene was found to relieve chronic and inflammatory pain, but without reducing reactions to acute pain, the type caused by a sudden injury. This is crucial: responses to injuries can be vital to our survival. The researchers hope that they'll be able to develop a drug to block the protein produced by HCN2, so the same effect can be seen in humans.
That concludes this month's research review. Again, apologies for the limited number of items covered, but I hope you'll agree there are some really interesting studies in there.
See you next month!
Sunday, 3 July 2011
MS research roundup July 2011
First this month, a very exciting discovery (for researchers and science geeks like me, anyway). Until now, MS researchers working in the very early stages of a project, when animals must be used, had only been able to use non-human primates when the illness had been artificially induced. Now a naturally occurring disease in Japanese macaques has been found by researchers in America.
This can give many more clues about the causes and development of the disease. It has already been discovered that the condition is associated with a herpes virus - linking in with previous research suggesting that MS in humans may be linked to previous infection with the herpes strain Epstein-Barr virus, which causes glandular fever (mononucleosis).
More herpes news. A Taiwanese research group have found that there may be an increased risk of MS in the year following an attack of shingles, caused by the herpes zoster virus, which also causes chickenpox. The study followed very large groups of people with and without shingles, and found that those with were almost 4 times as likely to develop MS as those without. This is a very interesting study, well designed and with a very large sample group: however it was limited almost entirely to Chinese patients, and Asian populations have considerably lower rates of MS than Western. This work needs to be repeated on other ethnic groups.
Now a piece of research that may not be news to many of us (including me!). American researchers have discovered that fatigue is the first symptom of MS in nearly 30% of cases, often months or even years before any demyelination can be observed. I can certainly remember multiple visits to my GP complaining of fatigue, when I was either told "But everyone gets tired." ("No this isn't NORMAL tired!") or offered anti-depressants. Not particularly helpful.
The group is planning studies to see if unexplained fatigue can be used as a predictor of MS, and another to see if people whose first MS symptom is fatigue differ in any other way from those who have multiple MS-related symptoms.
It's been known for some time that women with MS who become pregnant have a higher than normal likelihood of experiencing a remission during pregnancy, and then experiencing a relapse after the birth. Now Canadian researchers have looked at the effect of MS on the actual pregnancy. They found overall that pregnancy was safe for women with MS, with no significant difference in gestational age, birth weight or type of birth (vaginal or caesarian).
Women with a greater level of disability were more likely to have adverse birth outcomes, but these did not reach statistically significant levels. The researchers also found that women with MS were more likely to be overweight, which is associated with greater risk during pregnancy and birth.
Now, on to treatments, and first some news about existing drugs. I've spoken before about the highly promising treatment Tysabri. Getting Tysabri carries the risk of developing the rare but severe condition progressive multifocal leukoencephalopathy (PML). PML is more likely if you have previous been exposed to (and therefore have antibodies to) anti-JC virus (JCV).
From now on, all patients in the EU will be tested for JCV antibody status before starting on Tysabri, and JCV antibody status will be included as a risk factor in Tysabri labelling. The risk of PML is still there, but it should be greatly reduced if people with antibodies to JCV do not take the drug.
Another existing treatment, of longer standing, is Copaxone(glatimer acetate). A group of Canadian researchers found that after a year patients receiving a new oral drug, teriflunomide, along with Copaxone, had only about 1/3 the number of lesions of a control group who took Copaxone plus a placebo tablet.
Teriflunomide has previously been tested with interferon: that trial also showed impressive results. It operates by stopping certain immune cells from dividing. This sounds like a very promising adjunct treatment. I hope it's trialled in larger groups, and against other therapies, as soon as possible.
American researchers have developed a very promising new approach to identifying the "self" proteins our bodies attack in auto-immune diseases like MS, rheumatoid arthritis and diabetes. Their new technique, called phage immunoprecipitation sequencing, allows the identification of any interaction between an autoantibody in the cerebrospinal fluid and the self-protein that drives the autoimmune response. This work is at very early stages, but could eventually be developed into diagnostic tests and even treatments.
Meanwhile, British researchers have discovered a synthetic chemical compound which both reduces the inflammatory response in the body and stimulates the body to produce interferon-beta, an anti-inflammatory molecule commonly given to people with MS. Together, these effects cause significant reduction of inflammation in an animal model of MS. The researchers also found that immune system cells from people with MS were more sensitive to the drug than immune system cells from people without the condition.
Excitingly, a joint research group from Britain and the USA have discovered a new molecule that they believe may lead to a drug able to repair myelin by stimulating stem cells. Again, this is very early stage research. Hopefully it will move to clinical trials and ultimately to some form of treatment, probably within 10-15 years. That would repay all the work that's being going on in myelin repair research.
So, that's what happened in June. Some really exciting news this time! See you, same place next month?
This can give many more clues about the causes and development of the disease. It has already been discovered that the condition is associated with a herpes virus - linking in with previous research suggesting that MS in humans may be linked to previous infection with the herpes strain Epstein-Barr virus, which causes glandular fever (mononucleosis).
More herpes news. A Taiwanese research group have found that there may be an increased risk of MS in the year following an attack of shingles, caused by the herpes zoster virus, which also causes chickenpox. The study followed very large groups of people with and without shingles, and found that those with were almost 4 times as likely to develop MS as those without. This is a very interesting study, well designed and with a very large sample group: however it was limited almost entirely to Chinese patients, and Asian populations have considerably lower rates of MS than Western. This work needs to be repeated on other ethnic groups.
Now a piece of research that may not be news to many of us (including me!). American researchers have discovered that fatigue is the first symptom of MS in nearly 30% of cases, often months or even years before any demyelination can be observed. I can certainly remember multiple visits to my GP complaining of fatigue, when I was either told "But everyone gets tired." ("No this isn't NORMAL tired!") or offered anti-depressants. Not particularly helpful.
The group is planning studies to see if unexplained fatigue can be used as a predictor of MS, and another to see if people whose first MS symptom is fatigue differ in any other way from those who have multiple MS-related symptoms.
It's been known for some time that women with MS who become pregnant have a higher than normal likelihood of experiencing a remission during pregnancy, and then experiencing a relapse after the birth. Now Canadian researchers have looked at the effect of MS on the actual pregnancy. They found overall that pregnancy was safe for women with MS, with no significant difference in gestational age, birth weight or type of birth (vaginal or caesarian).
Women with a greater level of disability were more likely to have adverse birth outcomes, but these did not reach statistically significant levels. The researchers also found that women with MS were more likely to be overweight, which is associated with greater risk during pregnancy and birth.
Now, on to treatments, and first some news about existing drugs. I've spoken before about the highly promising treatment Tysabri. Getting Tysabri carries the risk of developing the rare but severe condition progressive multifocal leukoencephalopathy (PML). PML is more likely if you have previous been exposed to (and therefore have antibodies to) anti-JC virus (JCV).
From now on, all patients in the EU will be tested for JCV antibody status before starting on Tysabri, and JCV antibody status will be included as a risk factor in Tysabri labelling. The risk of PML is still there, but it should be greatly reduced if people with antibodies to JCV do not take the drug.
Another existing treatment, of longer standing, is Copaxone(glatimer acetate). A group of Canadian researchers found that after a year patients receiving a new oral drug, teriflunomide, along with Copaxone, had only about 1/3 the number of lesions of a control group who took Copaxone plus a placebo tablet.
Teriflunomide has previously been tested with interferon: that trial also showed impressive results. It operates by stopping certain immune cells from dividing. This sounds like a very promising adjunct treatment. I hope it's trialled in larger groups, and against other therapies, as soon as possible.
American researchers have developed a very promising new approach to identifying the "self" proteins our bodies attack in auto-immune diseases like MS, rheumatoid arthritis and diabetes. Their new technique, called phage immunoprecipitation sequencing, allows the identification of any interaction between an autoantibody in the cerebrospinal fluid and the self-protein that drives the autoimmune response. This work is at very early stages, but could eventually be developed into diagnostic tests and even treatments.
Meanwhile, British researchers have discovered a synthetic chemical compound which both reduces the inflammatory response in the body and stimulates the body to produce interferon-beta, an anti-inflammatory molecule commonly given to people with MS. Together, these effects cause significant reduction of inflammation in an animal model of MS. The researchers also found that immune system cells from people with MS were more sensitive to the drug than immune system cells from people without the condition.
Excitingly, a joint research group from Britain and the USA have discovered a new molecule that they believe may lead to a drug able to repair myelin by stimulating stem cells. Again, this is very early stage research. Hopefully it will move to clinical trials and ultimately to some form of treatment, probably within 10-15 years. That would repay all the work that's being going on in myelin repair research.
So, that's what happened in June. Some really exciting news this time! See you, same place next month?
Wednesday, 15 June 2011
Smoke gets in your eyes - or is it Optic Neuritis?
I've noticed, among people that I know, that a lot of people with MS seem to smoke. I've often wondered whether it's the smoking that causes their MS, or having MS that makes them not want to give up. Or is there a third factor that's related to both of them?
Incidentally, I should declare a conflict of interest here, being on day 3 of giving up smoking. If I should come over all sanctimonious ex-smoker, you have my full permission to kill me, OK?
Smoking is now accepted as a risk factor for MS (as is passive smoking), and it appears that it may also make the disease course worse, both clinically and on MRI. Heavier smoking seems to increase the risk. Smoking is also associated with the type of MS someone has. Current smokers are more likely to have primary progressive MS, and smokers progress from relapsing-remitting MS to secondary progressive MS faster than those who have never smoked.
In addition, people with MS who smoke are more likely to have other auto-immune conditions, such as lupus, Crohn's disease, or pernicious anaemia. It seems that nicotine can precipitate the inflammatory response. Having more than one condition affects the length of time between symptom onset and diagnosis, disability progression, and health-related quality of life.
Smoking is associated with disruption of the blood-brain barrier, which will make it easier for immune system cells to get through and cause damage, with more lesions, and with more atrophy (wasting away) of the brain.
Do smokers with MS get the same encouragement as others to give up? Several friends with MS have told me of their GPs saying, in as many words:
Many people with MS also smoke those special herbal cigarettes for people with MS.
Could the smoking of cannabis by people with MS (most often for the relief of pain or spasticity, or to help with sleep) be linked to the high levels of cigarette smoking? After all, many cannabis users smoke cigarettes.
So what conclusions can we reach? Yes, smoking is a risk factor for MS - as it is for many other conditions. There's no question that it would be sensible for us not to start smoking, and if we have started, to stop. But we're not necessarily sensible. There are all sorts of reasons people smoke, that can outweigh possible, statistical risks.
I'm not going to tell anyone to give up smoking. I smoked for many years when I was fully aware of the risks - not just for my MS, but everything else too. I'm giving up now because I think it's finally the right time, for me. We all need the facts though, to be able to make an informed decision about risks and benefits.
Now, back to Smoke Gets In Your Eyes:
Incidentally, I should declare a conflict of interest here, being on day 3 of giving up smoking. If I should come over all sanctimonious ex-smoker, you have my full permission to kill me, OK?
Smoking is now accepted as a risk factor for MS (as is passive smoking), and it appears that it may also make the disease course worse, both clinically and on MRI. Heavier smoking seems to increase the risk. Smoking is also associated with the type of MS someone has. Current smokers are more likely to have primary progressive MS, and smokers progress from relapsing-remitting MS to secondary progressive MS faster than those who have never smoked.
In addition, people with MS who smoke are more likely to have other auto-immune conditions, such as lupus, Crohn's disease, or pernicious anaemia. It seems that nicotine can precipitate the inflammatory response. Having more than one condition affects the length of time between symptom onset and diagnosis, disability progression, and health-related quality of life.
Smoking is associated with disruption of the blood-brain barrier, which will make it easier for immune system cells to get through and cause damage, with more lesions, and with more atrophy (wasting away) of the brain.
Do smokers with MS get the same encouragement as others to give up? Several friends with MS have told me of their GPs saying, in as many words:
In your situation, if smoking makes you happy - carry on!In other cases, people may feel that they're buggered physically anyway, so why go through the stress of giving up? One friend told me that she resented being told to give up smoking, something she enjoyed doing, when she'd already lost so much.
Many people with MS also smoke those special herbal cigarettes for people with MS.
Could the smoking of cannabis by people with MS (most often for the relief of pain or spasticity, or to help with sleep) be linked to the high levels of cigarette smoking? After all, many cannabis users smoke cigarettes.
So what conclusions can we reach? Yes, smoking is a risk factor for MS - as it is for many other conditions. There's no question that it would be sensible for us not to start smoking, and if we have started, to stop. But we're not necessarily sensible. There are all sorts of reasons people smoke, that can outweigh possible, statistical risks.
I'm not going to tell anyone to give up smoking. I smoked for many years when I was fully aware of the risks - not just for my MS, but everything else too. I'm giving up now because I think it's finally the right time, for me. We all need the facts though, to be able to make an informed decision about risks and benefits.
Now, back to Smoke Gets In Your Eyes:
(Smoke Gets In Your Eyes is a classic song, a standard, and very many people have recorded it. But being the age I am, this is the version I most remember, and one I love.)
Wednesday, 1 June 2011
MS research roundup June 2011
This month's research roundup includes several studies on treatments for MS, particularly Natalizumab (Tysabri). As usual, I'll do my best to arrange the post with risk factors for MS first, then symptoms, diagnosis and treatment.
It's still pretty obscure what causes MS, but researchers in the USA may have gone some way to finding out. It has long been suspected that there is a genetic component to MS, but the relationship is not simple. This study looked at the way a range of environmental factors such as metabolism and Vitamin D-3 levels interacted with four genes (interleukin-7 receptor-alpha, interleukin-2 receptor-alpha, MGAT1 and CTLA-4) to affect certain proteins that regulate the disease. They found that adding Vitamin D-3 or a simple sugar called GlcNAc could help to restore proteins to their normal functions. Sounds like a very promising line of research, and one that could potentially solve the mystery of MS causation.
Spanish researchers have looked at all previous studies investigating links between having been previously infected with Epstein-Barr virus (which causes glandular fever / mononucleosis) and MS, and confirmed that having antibodies to the virus means a higher likelihood of developing MS. The timing of the infection (how long ago) was not relevant. Further research is needed, though - ideally following a group of people from before any of them develop MS symptoms.
I've observed in the past that a high proportion of people with MS seem to be (cigarette!) smokers. A group of Swedish researchers have found that smoking interacts with two genetic factors, having a gene called human leukocyte antigen DRB1*15 and not having human leukocyte antigen A*02.Smokers with both genetic risk factors were 13.5 times as likely to develop MS as non-smokers with neither. The researchers believe that cigarette smoke acts in the lungs to "prime" the immune system and make MS more likely.
Many of us who have MS are fully aware that stressful life events can make our symptoms worse. But can stress cause MS in the first place? No, say researchers from the USA and Norway. They used data from two study of nurses' health, which started in 1976 and 1989. Participants were asked to report their level of stress at work and at home. The researchers found no link between self-reported stress and MS risk. As ever though, more research is needed.
We are used to MRI imaging being used to identify MS lesions, but Italian researchers have found that people with MS also experience atrophy (wasting away) of the brain and spinal cord. The amount of atrophy is linked to the degree of disability. They suggest that in the future MRI could be used to monitor response to treatment.
Now on to treatments, and as I said above several studies on Tysabri have reported this month. There have (quite rightly) been concerns about the risk of developing the potentially fatal brain infection progressive multifocal leukoencephalopathy (PML) while using Tysabri. PML is caused by the reactivation of a virus called Polyomavirus JC (JC virus) in the nervous system. JC virus is carried by many people, and is harmless unless the person has lowered immunity.
A number of different research groups gave conference reports on Tysabri and PML. One group has developed a blood test to check whether there has been previous infection with JC virus: it's thought that these patients will be at higher risk of PML when taking Tysabri, a risk that they must take into account when deciding whether or not to take the medication. Another study confirmed that having been on another immunosuppressant therapy at any point before Tysabri increases the risk of PML.
Although PML is unquestionably serious, survival rates among those who develop it while on Tysabri seem to be better than those with other conditions (such as AIDS), possibly due to the development of modern anteretroviral drugs. Most people with MS who develop PML will survive.
People of African descent who get MS typically have a more severe disease course than average, and are less likely to respond to treatment with interferon-beta. American researchers looked at data for Tysabri use in this group of patients, and confirmed that it worked well, reducing relapse rate by an average of 60% and lesion accumulation by 79%.
Because of the risk of PML, some doctors recommend breaks in treatment with Tysabri. A group of researchers in Canada found that disease activity (measured by relapse rate and lesion growth) returned very rapidly after Tysabri was discontinued, whether or not another treatment was started.
Two studies, in Holland and Germany, followed women who became pregnant while taking Tysabri. Both studies were small, as they included only accidental pregnancies: at the moment women on Tysabri are advised not to get pregnant. Out of a total of 37 pregnancies, 28 were completely normal, 5 ended in miscarriage and one woman had a termination: one child was born with an extra finger. These data are promising, but a much larger study is needed before it can be confirmed that Tysabri is safe in pregnancy.
The increased risk of developing PML while on Tysabri, which is highly successful at slowing disease progression, illustrates the need to balance the risks and benefits of any treatment. Australian researchers have found that MS patients are often prepared to take more risks than their doctors in terms of possible side-effects. This illustrates the importance of healthcare professionals listening to the priorities and views of their patients.
Now on to other treatments. The first treatment for MS, interferon-B, was developed and trialled in the late 1980s. A group of American researchers have tracked down participants in the original trials to see howthey had fared since then. The researchers found that those who received interferon-B were only half as likely to have died as those who received a placebo. It's not clear yet what accounts for this difference, as all the participants were likely to have been on other drugs subsequently. A very significant difference though, and one that needs to be investigated further.
There have been concerns about side-effects from the new oral treatment fingolimod (Gilenya), including a risk of lowered heart rate and a condition called AV block. An American study found that these effects were transient, and usually resolve within six hours without treatment. People should be screened for heart abnormalities before taking Gilenya, though, and carefully observed after the first dose.
Spanish researchers have found that Viagra dramatically reduces symptoms in animals with a condition similar to MS. They are hoping to begin clinical trials soon. I'll be interested to see the results. MS can cause sexual dysfunction, and many men with MS are prescribed Viagra. I've not heard of any of them having "miracle cures" from MS...
It's been found that less than half of people with MS are still consistently taking their disease-modifying drugs after 2 years, possibly because of side effects or people perceiving no benefit from them. What is needed, as we all know, is a cure. Excitingly, American and Chinese scientists have succeeded in making human stem cells convert into astrocytes, the cell type which promotes myelination. In my view, stem cell research is what will eventually provide a cure for MS, and this is a major step forward.
Finally - and, I'm afraid, rather depressingly - some British research into the use of antidepressants in MS. Although treatment with antidepressants was always preferable to no treatment, there was no definite evidence that quality of life was improved as a result. Depression is a very common symptom of MS, and it's important that people feel they can get an effective treatment when it's needed. More research is needed to discover whether some anti-depressants are more helpful in MS than others.
So, it was another bumper bundle this month. Does the reporting of research results slow down in the summer? Let you know in July!
It's still pretty obscure what causes MS, but researchers in the USA may have gone some way to finding out. It has long been suspected that there is a genetic component to MS, but the relationship is not simple. This study looked at the way a range of environmental factors such as metabolism and Vitamin D-3 levels interacted with four genes (interleukin-7 receptor-alpha, interleukin-2 receptor-alpha, MGAT1 and CTLA-4) to affect certain proteins that regulate the disease. They found that adding Vitamin D-3 or a simple sugar called GlcNAc could help to restore proteins to their normal functions. Sounds like a very promising line of research, and one that could potentially solve the mystery of MS causation.
Spanish researchers have looked at all previous studies investigating links between having been previously infected with Epstein-Barr virus (which causes glandular fever / mononucleosis) and MS, and confirmed that having antibodies to the virus means a higher likelihood of developing MS. The timing of the infection (how long ago) was not relevant. Further research is needed, though - ideally following a group of people from before any of them develop MS symptoms.
I've observed in the past that a high proportion of people with MS seem to be (cigarette!) smokers. A group of Swedish researchers have found that smoking interacts with two genetic factors, having a gene called human leukocyte antigen DRB1*15 and not having human leukocyte antigen A*02.Smokers with both genetic risk factors were 13.5 times as likely to develop MS as non-smokers with neither. The researchers believe that cigarette smoke acts in the lungs to "prime" the immune system and make MS more likely.
Many of us who have MS are fully aware that stressful life events can make our symptoms worse. But can stress cause MS in the first place? No, say researchers from the USA and Norway. They used data from two study of nurses' health, which started in 1976 and 1989. Participants were asked to report their level of stress at work and at home. The researchers found no link between self-reported stress and MS risk. As ever though, more research is needed.
We are used to MRI imaging being used to identify MS lesions, but Italian researchers have found that people with MS also experience atrophy (wasting away) of the brain and spinal cord. The amount of atrophy is linked to the degree of disability. They suggest that in the future MRI could be used to monitor response to treatment.
Now on to treatments, and as I said above several studies on Tysabri have reported this month. There have (quite rightly) been concerns about the risk of developing the potentially fatal brain infection progressive multifocal leukoencephalopathy (PML) while using Tysabri. PML is caused by the reactivation of a virus called Polyomavirus JC (JC virus) in the nervous system. JC virus is carried by many people, and is harmless unless the person has lowered immunity.
A number of different research groups gave conference reports on Tysabri and PML. One group has developed a blood test to check whether there has been previous infection with JC virus: it's thought that these patients will be at higher risk of PML when taking Tysabri, a risk that they must take into account when deciding whether or not to take the medication. Another study confirmed that having been on another immunosuppressant therapy at any point before Tysabri increases the risk of PML.
Although PML is unquestionably serious, survival rates among those who develop it while on Tysabri seem to be better than those with other conditions (such as AIDS), possibly due to the development of modern anteretroviral drugs. Most people with MS who develop PML will survive.
People of African descent who get MS typically have a more severe disease course than average, and are less likely to respond to treatment with interferon-beta. American researchers looked at data for Tysabri use in this group of patients, and confirmed that it worked well, reducing relapse rate by an average of 60% and lesion accumulation by 79%.
Because of the risk of PML, some doctors recommend breaks in treatment with Tysabri. A group of researchers in Canada found that disease activity (measured by relapse rate and lesion growth) returned very rapidly after Tysabri was discontinued, whether or not another treatment was started.
Two studies, in Holland and Germany, followed women who became pregnant while taking Tysabri. Both studies were small, as they included only accidental pregnancies: at the moment women on Tysabri are advised not to get pregnant. Out of a total of 37 pregnancies, 28 were completely normal, 5 ended in miscarriage and one woman had a termination: one child was born with an extra finger. These data are promising, but a much larger study is needed before it can be confirmed that Tysabri is safe in pregnancy.
The increased risk of developing PML while on Tysabri, which is highly successful at slowing disease progression, illustrates the need to balance the risks and benefits of any treatment. Australian researchers have found that MS patients are often prepared to take more risks than their doctors in terms of possible side-effects. This illustrates the importance of healthcare professionals listening to the priorities and views of their patients.
Now on to other treatments. The first treatment for MS, interferon-B, was developed and trialled in the late 1980s. A group of American researchers have tracked down participants in the original trials to see howthey had fared since then. The researchers found that those who received interferon-B were only half as likely to have died as those who received a placebo. It's not clear yet what accounts for this difference, as all the participants were likely to have been on other drugs subsequently. A very significant difference though, and one that needs to be investigated further.
There have been concerns about side-effects from the new oral treatment fingolimod (Gilenya), including a risk of lowered heart rate and a condition called AV block. An American study found that these effects were transient, and usually resolve within six hours without treatment. People should be screened for heart abnormalities before taking Gilenya, though, and carefully observed after the first dose.
Spanish researchers have found that Viagra dramatically reduces symptoms in animals with a condition similar to MS. They are hoping to begin clinical trials soon. I'll be interested to see the results. MS can cause sexual dysfunction, and many men with MS are prescribed Viagra. I've not heard of any of them having "miracle cures" from MS...
It's been found that less than half of people with MS are still consistently taking their disease-modifying drugs after 2 years, possibly because of side effects or people perceiving no benefit from them. What is needed, as we all know, is a cure. Excitingly, American and Chinese scientists have succeeded in making human stem cells convert into astrocytes, the cell type which promotes myelination. In my view, stem cell research is what will eventually provide a cure for MS, and this is a major step forward.
Finally - and, I'm afraid, rather depressingly - some British research into the use of antidepressants in MS. Although treatment with antidepressants was always preferable to no treatment, there was no definite evidence that quality of life was improved as a result. Depression is a very common symptom of MS, and it's important that people feel they can get an effective treatment when it's needed. More research is needed to discover whether some anti-depressants are more helpful in MS than others.
So, it was another bumper bundle this month. Does the reporting of research results slow down in the summer? Let you know in July!
Monday, 2 May 2011
MS research roundup May 2011
Yet again, there's been lots of interesting research published this month. Some of it is quite technical, but I'll do my best to translate it into English - depends on me working out what it means myself though!
I've written in previous posts about the link between lack of exposure to sunlight and getting MS. It's also been known for a while that people with antibodies to Epstein-Barr virus, which causes glandular fever (mononucleosis), are more likely to get MS than people without. British researchers looked at the two together, to see if their data could help explain the variance of the disease across the UK. They found that adding the effects of Epstein-Barr and exposure to sunlight together explained more of the differences than exposure to sunlight alone. They suspect that exposure to Epstein-Barr may lead to an abnormal response to vitamin D deficiency.
Still on Epstein-Barr virus: a British research team has been investigating the mechanism which causes the virus to increase MS risk. They were looking at epigenetic changes in DNA. Epigenetic changes are non-genetic changes leading to the genes behaving (expressing themselves) differently. These effects can last for several generation. There is now convincing evidence for an epigenetic component to MS. Effects have been found for mother and child, grandparent and child, and early life (birth month). This study found many locations on the genome different before and after infection with Epstein-Barr.
I've also previously mentioned that people with one autoimmune condition seem more likely to have another, and various pieces of research are being done to confirm this with people with MS. Spanish researchers investigated people with MS to see whether coeliac disease was more common in them than in the general population. Coeliac disease is an autoimmune disease of the small intestine, caused by a reaction to proteins in wheat, barley and rye. Worldwide, about 1-2% of the general population have coeliac disease. This study found that 11% of people with MS had coeliac disease, and a very high prevalence of 32% among their first degree relatives.
This gives me a chance to air one of my pet theories, backed by nothing at all that I'm aware of except personal anecdotes. If you ask people with MS, you'll find an awful lot of them have autoimmune conditions in their immediate family - lupus, rheumatoid arthritis, type 1 (juvenile onset) diabetes, and so on. My dad had Parkinson's disease, which is also autoimmune. I suspect that we may inherit a tendency to get autoimmune conditions, and what particular one or ones we get is then down to viruses, environmental factors and so on. Are any research teams reading this...?
Two different research teams, one in the USA and one in Switzerland, have found that an immune system molecule called GM-CSF plays a central role in the inflammation of the central nervous system that occurs in conditions like MS. The research was in animals bred to have a condition that mimics MS. If some way can be found of blocking GM-CSF activity, this would be a possible treatment for MS.
Previous studies have shown that white blood cells called leukocytes enter the central nervous system and play a major part in causing the damage contributing to MS symptoms. Other studies have shown that molecules called MMPs help the leukocytes enter the CNS. Now Canadian researchers have found that a molecular switch called EMMPRIN plays an important part in this process. They found that inhibiting the production of EMMPRIN reduced MS-like symptoms in mice. They also found that EMMPRIN is significantly raised in brain lesions in MS patients. EMMPRIN is another new possible target for MS treatments.
One of the major concerns with MS, and other conditions like it, is uncertainty: not knowing how far, and how fast, disability will progress. Now researchers from the Mayo Clinic in the USA have discovered what they say is a way of predicting how disability will progress in people with secondary progressive MS (SPMS). They found that people with SPMS and a faster disability rate had higher levels of an immune system molecule called immunoglobulin G in their cerebrospinal fluid. They concluded that if someone with SPMS has a higher level of IgG, it is likely that they will have a faster rate of disability.
But do we want to know? Of course, it would be lovely to know we're going to have a slow rate of progression. But there's the risk of learning the opposite...not sure it's a test I would want.
And before moving on to medical treatments, one natural therapy that's sadly not open to all of us. It's already known that women tend to have remissions in pregnancy and relapses following childbirth. German researchers have now found that the (possibly hormonal) protection of pregnancy is extended if the baby is exclusively breastfed. However, breastfeeding can't be combined with disease-modifying therapies, so previously the mother had to decide without clear evidence to support their choice. More research is needed, though.
So, on to medical treatments. First, some news about an existing treatment, interferon-beta, from a research group in Spain. HHV-6 is a form of the human herpes virus, and is present in a high proportion of the human population. It causes the childhood fever known as exanthem subitum or roseola infantum. Although it then remains in the body for life, it is symptom-free. These researchers found that people with antibodies to HHV-6 in their blood had more relapses and responded less well to interferon-beta than those without.
There has been a lot of media coverage over the UK approval of Gilenya (fingolimod), the first oral disease modifying treatment for MS. This is only one of the many possible links. The media (perhaps understandably, given their general audience) didn't give much stress though to the conditions under which it will be given. I think they're important. Gilenya is for people with aggressive relapsing remitting MS (RRMS), and will only be prescribed when the standard therapies have failed. This is because of possible serious side effects, including reduced heart rate and eye problems. So for most people with RRMS, I'm afraid it's injections as before!
Now onto newer disease modifiers, still in the research stage. Laquinimod is another oral treatment, and has just completed a large phase 3 clinical trial with over 1100 patients in 26 countries. The Italian researchers found that those on laquinimod had 23% fewer relapses, 36% less disability progression, and 33% less brain atrophy than those on a placebo. It seems to be well tolerated and have a good safety rate. A promising treatment, but a pity from a personal viewpoint that yet again it's for people with RRMS!
Another phase 3 clinical trial reporting recently investigated the use of an oral drug called BG-12 (dimethyl fumarate) in more that 1200 people with RRMS. Study participants who were taking BG-12 were significantly less likely to have had a relapse after 2 years than those on a placebo. There was also a significant reduction in the annual relapse rate, the number of new or enlarging brain lesions, and the rate of disability progression. Unfortunately, BG-12 has to be taken 2 or 3 times a day, which could reduce compliance. If a sustained release version can be developed, this could be a really useful treatment for people with RRMS.
Olesoxime is a proposed new treatment for spinal muscular atrophy (SMA), a genetic disease characterised by progressive degeneration of muscle neurones. As MS attacks the same part of the neurone as does SMA, French researchers have been investigating whether olesoxime could also be beneficial in MS. They suspect that while acute relapses are caused by inflammation of the CNS, progression is caused by degeneration. The research has not yet reached human volunteers, but looks promising.
Finally, reports on research into treatment of two symptoms of MS. Ampyra (dalfampridine) is a drug designed to help with mobility problems. It has been approved in the USA and various other countries, but not yet in the UK due to concerns over side effects. Detailed analyses of all the Ampyra trials that have taken place show that there is increased risk of seizures, and also of urinary tract infections - always a concern for people with MS, particularly those on disease modifiers which depress the immune system.
Finally for this month, a phase 2 clinical trial is beginning on a new drug for neuropathic pain. It's hard to believe that it was as recently as the 1980s when MS pain was acknowledged. Those of us with chronic neuropathic pain know how severe and debilitating it can be, and how badly it can affect quality of life. The trial for the new drug, AVP-923, will measure levels of pain, fatigue, sleep quality and cognition. Sounds very interesting - I look forward to seeing the results!
That's it for May. Another long post I'm afraid, but they will keep doing this interesting research!
I've written in previous posts about the link between lack of exposure to sunlight and getting MS. It's also been known for a while that people with antibodies to Epstein-Barr virus, which causes glandular fever (mononucleosis), are more likely to get MS than people without. British researchers looked at the two together, to see if their data could help explain the variance of the disease across the UK. They found that adding the effects of Epstein-Barr and exposure to sunlight together explained more of the differences than exposure to sunlight alone. They suspect that exposure to Epstein-Barr may lead to an abnormal response to vitamin D deficiency.
Still on Epstein-Barr virus: a British research team has been investigating the mechanism which causes the virus to increase MS risk. They were looking at epigenetic changes in DNA. Epigenetic changes are non-genetic changes leading to the genes behaving (expressing themselves) differently. These effects can last for several generation. There is now convincing evidence for an epigenetic component to MS. Effects have been found for mother and child, grandparent and child, and early life (birth month). This study found many locations on the genome different before and after infection with Epstein-Barr.
I've also previously mentioned that people with one autoimmune condition seem more likely to have another, and various pieces of research are being done to confirm this with people with MS. Spanish researchers investigated people with MS to see whether coeliac disease was more common in them than in the general population. Coeliac disease is an autoimmune disease of the small intestine, caused by a reaction to proteins in wheat, barley and rye. Worldwide, about 1-2% of the general population have coeliac disease. This study found that 11% of people with MS had coeliac disease, and a very high prevalence of 32% among their first degree relatives.
This gives me a chance to air one of my pet theories, backed by nothing at all that I'm aware of except personal anecdotes. If you ask people with MS, you'll find an awful lot of them have autoimmune conditions in their immediate family - lupus, rheumatoid arthritis, type 1 (juvenile onset) diabetes, and so on. My dad had Parkinson's disease, which is also autoimmune. I suspect that we may inherit a tendency to get autoimmune conditions, and what particular one or ones we get is then down to viruses, environmental factors and so on. Are any research teams reading this...?
Two different research teams, one in the USA and one in Switzerland, have found that an immune system molecule called GM-CSF plays a central role in the inflammation of the central nervous system that occurs in conditions like MS. The research was in animals bred to have a condition that mimics MS. If some way can be found of blocking GM-CSF activity, this would be a possible treatment for MS.
Previous studies have shown that white blood cells called leukocytes enter the central nervous system and play a major part in causing the damage contributing to MS symptoms. Other studies have shown that molecules called MMPs help the leukocytes enter the CNS. Now Canadian researchers have found that a molecular switch called EMMPRIN plays an important part in this process. They found that inhibiting the production of EMMPRIN reduced MS-like symptoms in mice. They also found that EMMPRIN is significantly raised in brain lesions in MS patients. EMMPRIN is another new possible target for MS treatments.
One of the major concerns with MS, and other conditions like it, is uncertainty: not knowing how far, and how fast, disability will progress. Now researchers from the Mayo Clinic in the USA have discovered what they say is a way of predicting how disability will progress in people with secondary progressive MS (SPMS). They found that people with SPMS and a faster disability rate had higher levels of an immune system molecule called immunoglobulin G in their cerebrospinal fluid. They concluded that if someone with SPMS has a higher level of IgG, it is likely that they will have a faster rate of disability.
But do we want to know? Of course, it would be lovely to know we're going to have a slow rate of progression. But there's the risk of learning the opposite...not sure it's a test I would want.
And before moving on to medical treatments, one natural therapy that's sadly not open to all of us. It's already known that women tend to have remissions in pregnancy and relapses following childbirth. German researchers have now found that the (possibly hormonal) protection of pregnancy is extended if the baby is exclusively breastfed. However, breastfeeding can't be combined with disease-modifying therapies, so previously the mother had to decide without clear evidence to support their choice. More research is needed, though.
So, on to medical treatments. First, some news about an existing treatment, interferon-beta, from a research group in Spain. HHV-6 is a form of the human herpes virus, and is present in a high proportion of the human population. It causes the childhood fever known as exanthem subitum or roseola infantum. Although it then remains in the body for life, it is symptom-free. These researchers found that people with antibodies to HHV-6 in their blood had more relapses and responded less well to interferon-beta than those without.
There has been a lot of media coverage over the UK approval of Gilenya (fingolimod), the first oral disease modifying treatment for MS. This is only one of the many possible links. The media (perhaps understandably, given their general audience) didn't give much stress though to the conditions under which it will be given. I think they're important. Gilenya is for people with aggressive relapsing remitting MS (RRMS), and will only be prescribed when the standard therapies have failed. This is because of possible serious side effects, including reduced heart rate and eye problems. So for most people with RRMS, I'm afraid it's injections as before!
Now onto newer disease modifiers, still in the research stage. Laquinimod is another oral treatment, and has just completed a large phase 3 clinical trial with over 1100 patients in 26 countries. The Italian researchers found that those on laquinimod had 23% fewer relapses, 36% less disability progression, and 33% less brain atrophy than those on a placebo. It seems to be well tolerated and have a good safety rate. A promising treatment, but a pity from a personal viewpoint that yet again it's for people with RRMS!
Another phase 3 clinical trial reporting recently investigated the use of an oral drug called BG-12 (dimethyl fumarate) in more that 1200 people with RRMS. Study participants who were taking BG-12 were significantly less likely to have had a relapse after 2 years than those on a placebo. There was also a significant reduction in the annual relapse rate, the number of new or enlarging brain lesions, and the rate of disability progression. Unfortunately, BG-12 has to be taken 2 or 3 times a day, which could reduce compliance. If a sustained release version can be developed, this could be a really useful treatment for people with RRMS.
Olesoxime is a proposed new treatment for spinal muscular atrophy (SMA), a genetic disease characterised by progressive degeneration of muscle neurones. As MS attacks the same part of the neurone as does SMA, French researchers have been investigating whether olesoxime could also be beneficial in MS. They suspect that while acute relapses are caused by inflammation of the CNS, progression is caused by degeneration. The research has not yet reached human volunteers, but looks promising.
Finally, reports on research into treatment of two symptoms of MS. Ampyra (dalfampridine) is a drug designed to help with mobility problems. It has been approved in the USA and various other countries, but not yet in the UK due to concerns over side effects. Detailed analyses of all the Ampyra trials that have taken place show that there is increased risk of seizures, and also of urinary tract infections - always a concern for people with MS, particularly those on disease modifiers which depress the immune system.
Finally for this month, a phase 2 clinical trial is beginning on a new drug for neuropathic pain. It's hard to believe that it was as recently as the 1980s when MS pain was acknowledged. Those of us with chronic neuropathic pain know how severe and debilitating it can be, and how badly it can affect quality of life. The trial for the new drug, AVP-923, will measure levels of pain, fatigue, sleep quality and cognition. Sounds very interesting - I look forward to seeing the results!
That's it for May. Another long post I'm afraid, but they will keep doing this interesting research!
Subscribe to:
Posts (Atom)